Reading time: ~ 4 min.

Software as a Medical Device: a booming market
Several reports on Software as a Medical Device (SWaaMD) market highlights very interesting growth trends. If you take a look at the last one issued by Industry Research you won't be disappointed by the forecasts on 2022-28 global sales you're going to find on it.
In particular, the European SWaaMD market was about 5,430 USD million in 2019 in 2019 and it is expected to reach 24,898 UDS million in 2027, with a Compound Annual Growth Rate of 21.6% (2020-27).
If you think about the reasons why this market shows this promising growing trends you will find probably the memories of the first COVID-19 pandemic wave and the shortage of empty beds in hospital wards worldwide.
The public health systems had to strengthen their digitalization to provide therapies to patients in particular due to the overload of the healthcare infrastructure.
The European SWaaMD market and the compliance with EU regulations
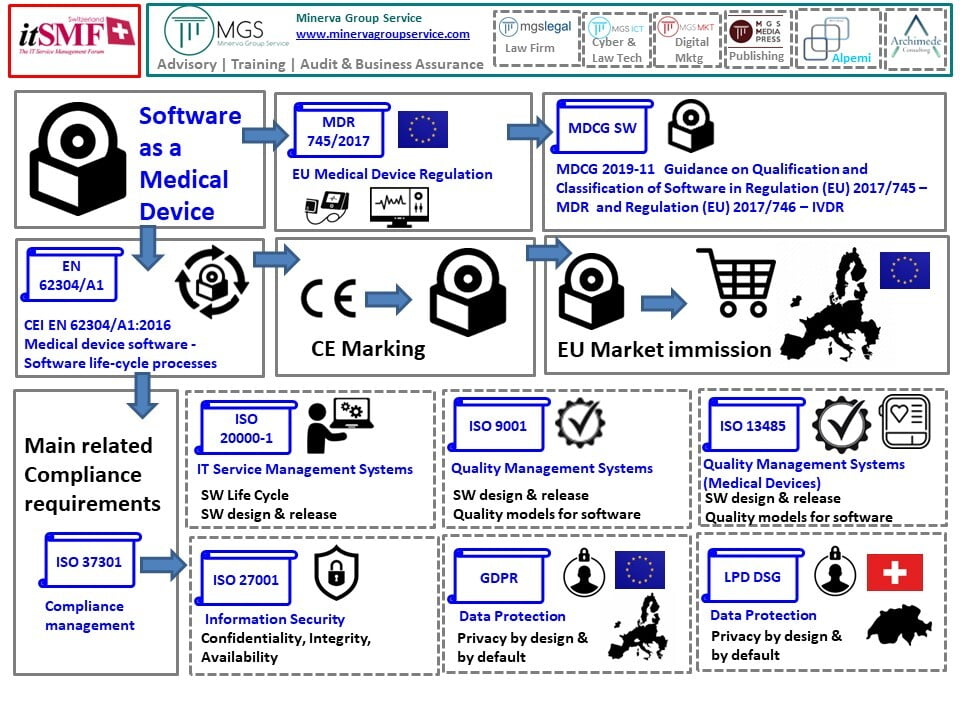
Even if to someone it could seems to be useless to remember, we want to remark that ALL the medical devices you plan to sell on the EU market must be CE marked.
Moreover software can also be classified as medical devices pursuant to EU Regulation 745/2017. In this case, we usually refer to «Software as a Medical Device» (SWaaMD) as outlined by the guidelines «MDCG 2019-11: Guidance on Qualification and Classification of Software in Regulation (EU) 2017/745 - MDR and Regulation (EU) 2017/746 - IVDR».
The exact evaluation on which are the laws you have to make sure you're compliant with to market your SWaaMD in EU it is critical to grant you the access to a growing market that is also regulated with restrictive privacy regulation (GDPR) in particular with sensitive personal data (e.g. the health condition of people).
Software as a Medical Device and the ISO Management Systems

As many other digital solutions, also on the Software as a Medical Device market we find some standards that are useful to define the main references you have to follow to develop products that are going to meet the needs and the expectations of the healthcare industry.
One of the most relevant standard is the IEC 62304:2006 known as a technical international standard about «Medical device software - software life cycle processes». But let focus a little bit more on the relationships you're going to find between ISO management systems and the mandatory requirements.
The ISO management systems and mandatory requirements
- IT Service ISO20000-1 about «Software life cycle»
- ISO9001 and ISO13485about «Quality for SW design and development»
- ISO/IEC25010:2011about «Models for SW quality»
- ISO27001 about «Information security for integrity, availability and confidentiality requirements»
- GDPR (as stated before) and LPD-DSG-LPD (Federal Act on Data Protection) for Switzerland, to be compliant with the privacy requirements refereed to by MDR 745/2017 itself.
We will for sure come back on this hot topic in the next posts we are going to publish on our posts.
If you have any question for us, feel free to drop us a line on our contact page.
See you soon again with more relevant content :) and don't forget to follow us on our social media channels.

