Reading time: ~ 2 min.
Software as a Medical Device (SaaMD): how to manage the requirements set by regulations?
We already had a look at Software as a Medical Device on our previous posts – here with a point of vue about data protection – and today we focus once again on SaaMD considering in particular innovation management and AI implementation related to this industry.
The production of medical devices can take advantage of the incredibile innovation brought by technology, but needs also to manage this development according to solid guidelines (as the ones delineated in ISO 56002 standard that can be applied to).
More and more software (SW) that are classified as medical devices pursuant to the EU MDR 2017/745 – see also the «MDCG 2019-11 Guidance on Qualification and Classification of Software in Regulation (EU) 2017/745 - MDR and Regulation (EU) 2017/746 - IVDR» – embed innovative solutions based on Artificial Intelligence.
The AI compliance with EU 2024/1689 regulation
Hence the need to manage compliance with the requirements set by the Regulation EU 24/1689 (commonly known as «AI Act») throughout the SW development cycle according to the IEC 62304: 2006 standard «Software for medical devices - Software life cycle processes »
The implementation of an Artificial Intelligence Management System according to the ISO 42001 standard supports effectively the development of innovative AI-based solutions also in the medical device industry; it can be also integrated with the following management systems:
ISO 13485 standard on «quality in the medical device sector»
ISO 20000-1 standard on «IT Services»
ISO 27001 standard on «Information security and data protection requirements» (GDPR).
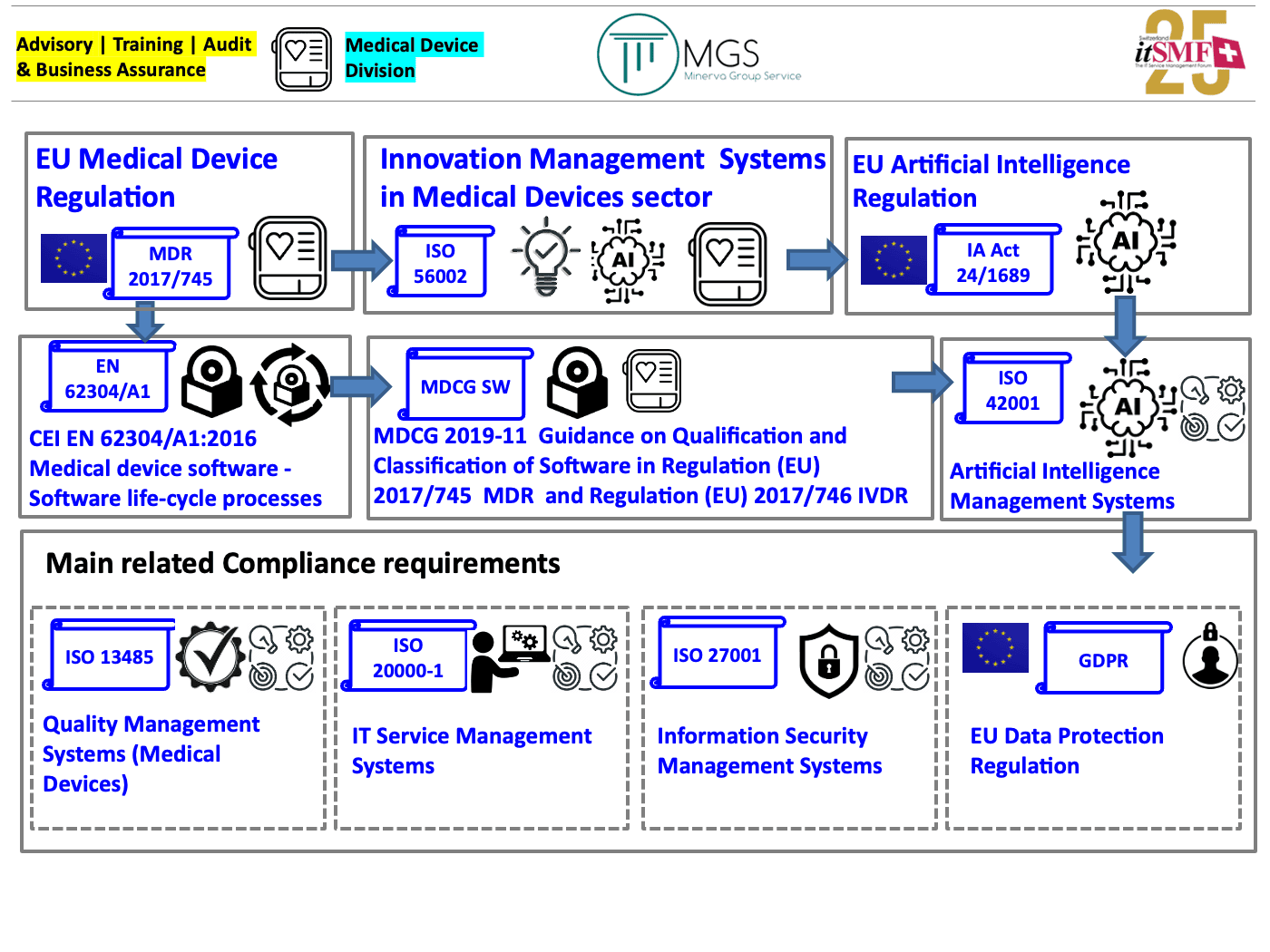
Our infographic on SaaMD related regulations and applicable ISO standards
To better figure out the «big picture» about the requirements set by regulations on SaaMD and the correlated ISO standards, take a look at our infographic:
If you want to keep you up-to-date with the most recent news on this topic, don't forget to subscribe to our newsletter: you will get a monthly update with the most relevant and valuable content from our experts!
Our sponsors
A special thanks to our Advanced Sponsors:





